Read the winnining entry of this years Science Blog Competition on autoimmunity and neurological diseases, by PhD student Stavroula Piliou.

Technological and scientific advancements have transformed how diseases are being treated. Many conditions that were fatal in the past are now manageable by treatment, providing near-normal life expectancies. This progress, however, has largely bypassed neurological disorders, which have grown to be the leading cause of disability worldwide. Focusing on the role of the immune system in these diseases provides a unique opportunity for breakthroughs in treatment.
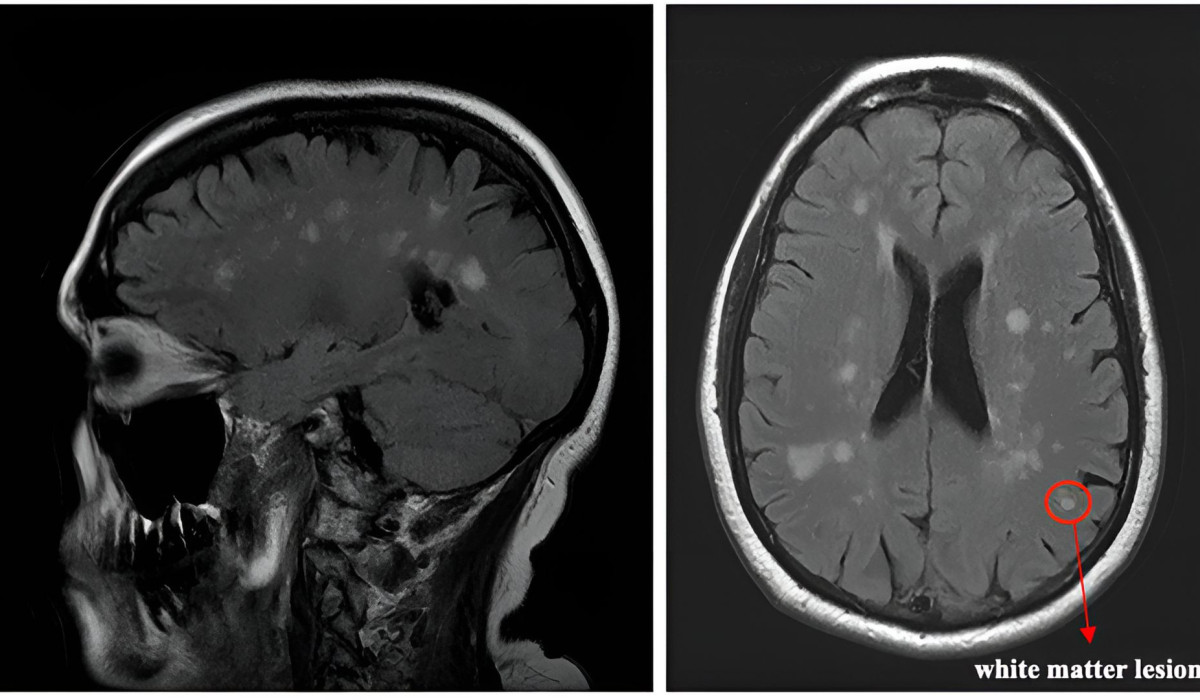
Multiple sclerosis (MS) is a great example of the immune system causing a neurological disease. MS is an unpredictable and often debilitating chronic autoimmune disease, where the immune system mistakenly attacks the cells (myelin) that support our nervous system. Over the past 20 years, more and more immunosuppressive treatments have been approved in an effort to minimise brain and spinal cord inflammation. For those forms of MS driven by immune reactions in the blood, the benefit of these treatments has been striking – decades of extra healthy life. However, for other forms of MS, the immune reaction is self-sustaining in the brain, and for these forms the creation of successful drugs remains difficult, due to the blood-brain-barrier (BBB) reducing the bioavailability of drugs in the brain. As a result, none of the available treatments can prevent disease progression once the reaction is self- sustaining in the brain.
This clinical need makes it important to target local immune reaction and focus on blockade of the proinflammatory responses within the brain. In this way, we could provide an anti-inflammatory and pro-regenerative immune environment. This is where my research comes in. For my project, I am utilising adeno-associated virus (AAV) gene delivery systems that can cross the BBB and produce biologic drugs within the brain. AAV, one of the most actively investigated gene therapy vehicles, is a single-stranded DNA virus that lacks immunogenicity and pathogenicity in humans. This can provide strong and sustained expression of any gene, delivered by AAV, making it an attractive long-term therapeutic option. My project builds on Prof. Adrian Liston's work, which has identified the importance of a white blood cell type in the brain called regulatory T cells (necessary for suppressing immune system activation and reducing inflammation). By delivering biologic drugs directly into the mouse brain to increase the number of these regulatory T cells, the group managed to promote neuroprotection, cortical tissue preservation and ongoing regeneration post-traumatic brain injury.
In my PhD research, I am re-engineering the AAV system to deliver other anti-inflammatory cytokines, pro-inflammatory blockers, and biologics that may promote neurological recovery and remyelination into the brain of mice with an experimental model of MS. I am studying their effects in MS during a relapse, during remission, in regeneration and in disease progression. Being inspired by the profound impact MS and other neurological diseases have on individuals’ quality of life, I hope my research begins to shine a light on the potential therapeutic value a gene delivery system may have, and to open new avenues in the search for revolutionary neuro-immunotherapies.